Exercise 8: Bayesian regression
We will now analyze data from the therapeutic clinical trial ALBI-ANRS 070 which compared the efficacy and tolerance of 3 anti-retroviral strategies among HIV-1 positive patients naive of any anti-retroviral treatment 1.
Load the data from the
albianrs_data.csvavailable here (ProTip: setstringsAsFactors = TRUEinread.delim())albi <- read.csv("albianrs_data.csv", stringsAsFactors = TRUE) summary(albi)## PatientID ViralLoad CD4 CD4t CD4sup500 ## ID112 : 7 Min. :1.699 Min. : 149.0 Min. :3.494 No :558 ## ID130 : 7 1st Qu.:2.042 1st Qu.: 377.0 1st Qu.:4.406 Yes:392 ## ID139 : 7 Median :2.712 Median : 465.0 Median :4.644 ## ID15 : 7 Mean :2.907 Mean : 491.8 Mean :4.664 ## ID156 : 7 3rd Qu.:3.692 3rd Qu.: 585.0 3rd Qu.:4.918 ## ID163 : 7 Max. :5.604 Max. :1318.0 Max. :6.025 ## (Other):908 ## Treatment Day Visit ## AZT+3TC:315 Min. : 0.00 Min. :0.000 ## d4t+ddI:322 1st Qu.: 29.00 1st Qu.:1.000 ## switch :313 Median : 84.00 Median :3.000 ## Mean : 86.19 Mean :2.946 ## 3rd Qu.:140.00 3rd Qu.:5.000 ## Max. :256.00 Max. :6.000 ##The following variables are available:
PatientID: Patient IDViralLoad: Plasma viral loadCD4: CD4 T-cell lymphocites rate (in cell/mm3)CD4t: transformed CD4 T-cell lymphocites rate (in cell/mm3) (CD4t=CD41/4)CD4sup500: binary variable indicating wether CD4 cell counts is above 500Treatment: Treatment group \(1\) (d4t + ddI), \(2\) (alternance) ou \(3\) (AZT + 3TC))Day: Day of visit (since inclusion)Visit: Visit number
Below is a quantitative summary of the characteristics of those variables.
| Variable | N = 9501 |
|---|---|
| ViralLoad | 2.71 (2.04, 3.69) |
| CD4 | 465 (377, 585) |
| CD4t | 4.64 (4.41, 4.92) |
| CD4sup500 | 392 (41%) |
| Treatment | |
| AZT+3TC | 315 (33%) |
| d4t+ddI | 322 (34%) |
| switch | 313 (33%) |
| Day | 84 (29, 140) |
| Visit | |
| 0 | 146 (15%) |
| 1 | 140 (15%) |
| 2 | 136 (14%) |
| 3 | 130 (14%) |
| 4 | 128 (13%) |
| 5 | 135 (14%) |
| 6 | 135 (14%) |
| 1 Median (IQR) or n (%) | |
NB: for simplicity, NA values have been omitted.
First, we want to explain the CD4 rate (as a transformed variable) from the viral load
Display
CD4tin scatterplot as a function of theViralLoadand color the points according toTreatment.library(ggplot2) library(MetBrewer) ggplot(albi, aes(y = CD4t, x = ViralLoad, color = Treatment)) + geom_point(alpha = 0.7) + MetBrewer::scale_color_met_d("Java") + theme_bw()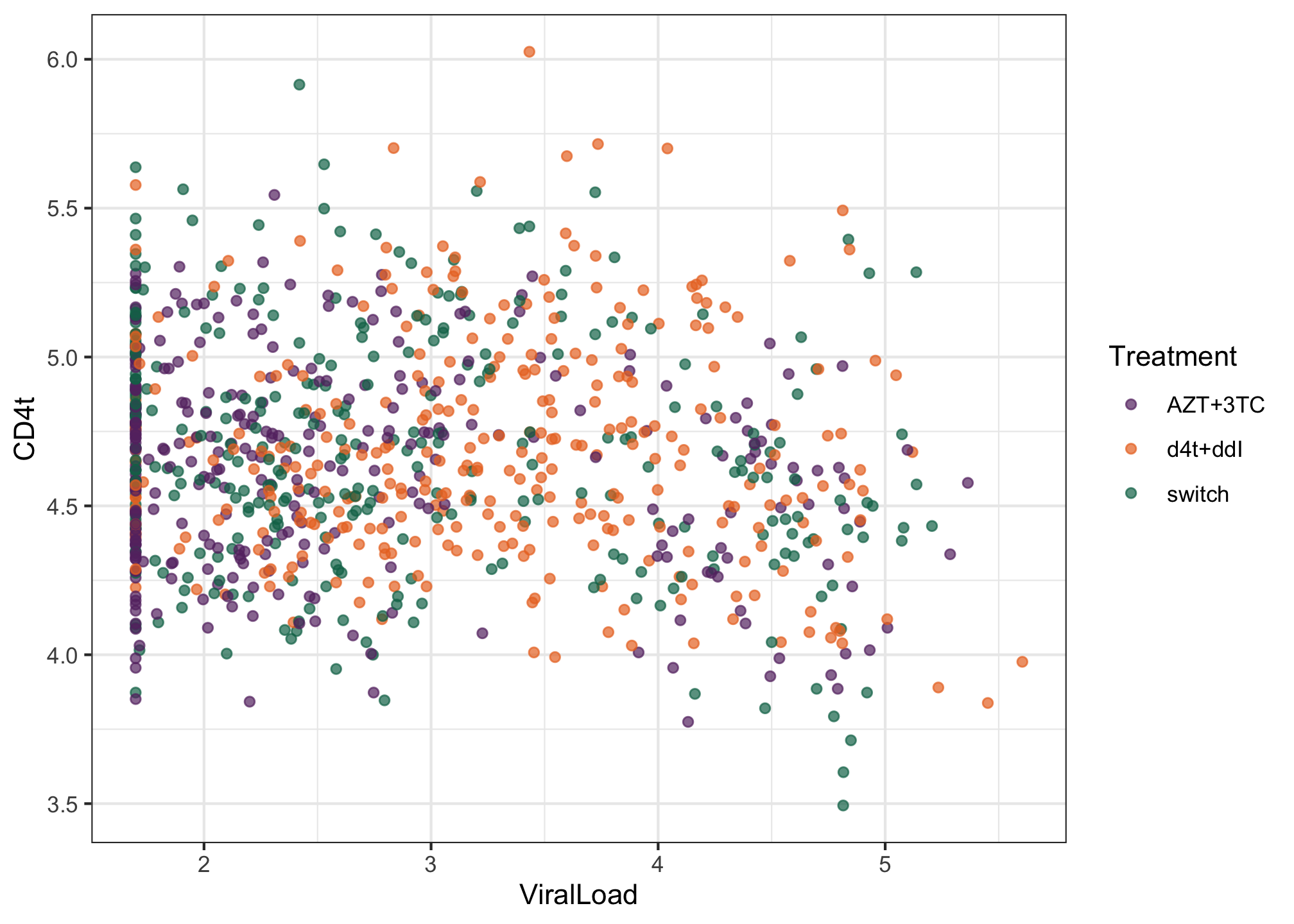
Write down the Bayesian mathematical model corresponding to a linear regression of
CD4tagainst theViralLoad.
I) Question of interest:
How does the viral load linearly explains the (transformed) CD4 T-cell rate ?
II) Sampling model:
Let \(CD4t_{i}\) be the \(i^{\text{th}}\) (transformed) CD4 T-cell rate \(i\) and \(VL_i^2\) the corresponding observed viral load: \[CD4t_i \overset{iid}{\sim} \mathcal{N}(\beta_0 + \beta_1 VL_i, \sigma^2)\] III) Priors: \[\beta_0\sim \mathcal{N}(0,100^2)\] \[\beta_1 \sim \mathcal{N}(0,100^2)\] \[\sigma^2 \sim InvGamma(0.001,0.001)\]Write the corresponding
BUGSmodel and save it in an external.txtfile.# Fixed effect linear regression Albi-ANRS model{ # Likelihood for (i in 1:N){ CD4t[i]~dnorm(mu[i], tau) mu[i] <- beta0 + beta1*VL[i] } # Priors beta0~dnorm(0,0.0001) beta1~dnorm(0,0.0001) # proper but very flat (vague: weakly informative) tau~dgamma(0.0001,0.0001) # proper but very flat (vague: weakly informative) # Parameters of interest sigma <- pow(tau, -0.5) }Create the corresponding
jagsobject in and generate a Monte Carlo sample of size 1000 for the 3 parameters of interestlibrary(rjags) albi_fixed_jags <- jags.model("albiBUGSmodel_fixed.txt", data = list(CD4t = albi$CD4t, N = nrow(albi), VL = albi$ViralLoad), n.chains = 3)## Compiling model graph ## Resolving undeclared variables ## Allocating nodes ## Graph information: ## Observed stochastic nodes: 950 ## Unobserved stochastic nodes: 3 ## Total graph size: 3195 ## ## Initializing modelres_albi_fixed <- coda.samples(model = albi_fixed_jags, variable.names = c("beta0", "beta1", "sigma"), n.iter = 1000)Before interpreting the results, check the convergence. What do you notice ?
library(coda) plot(res_albi_fixed)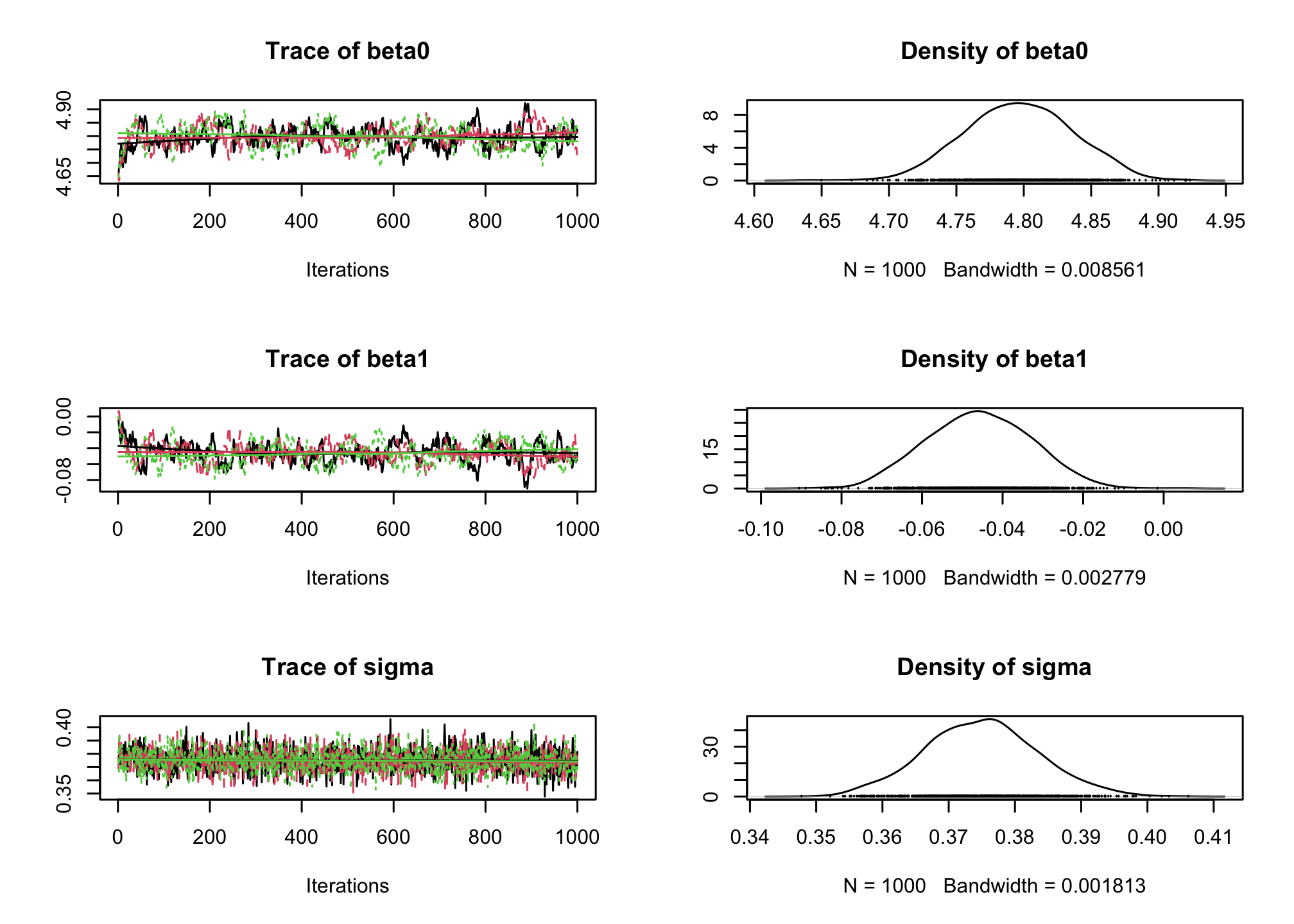
gelman.plot(res_albi_fixed)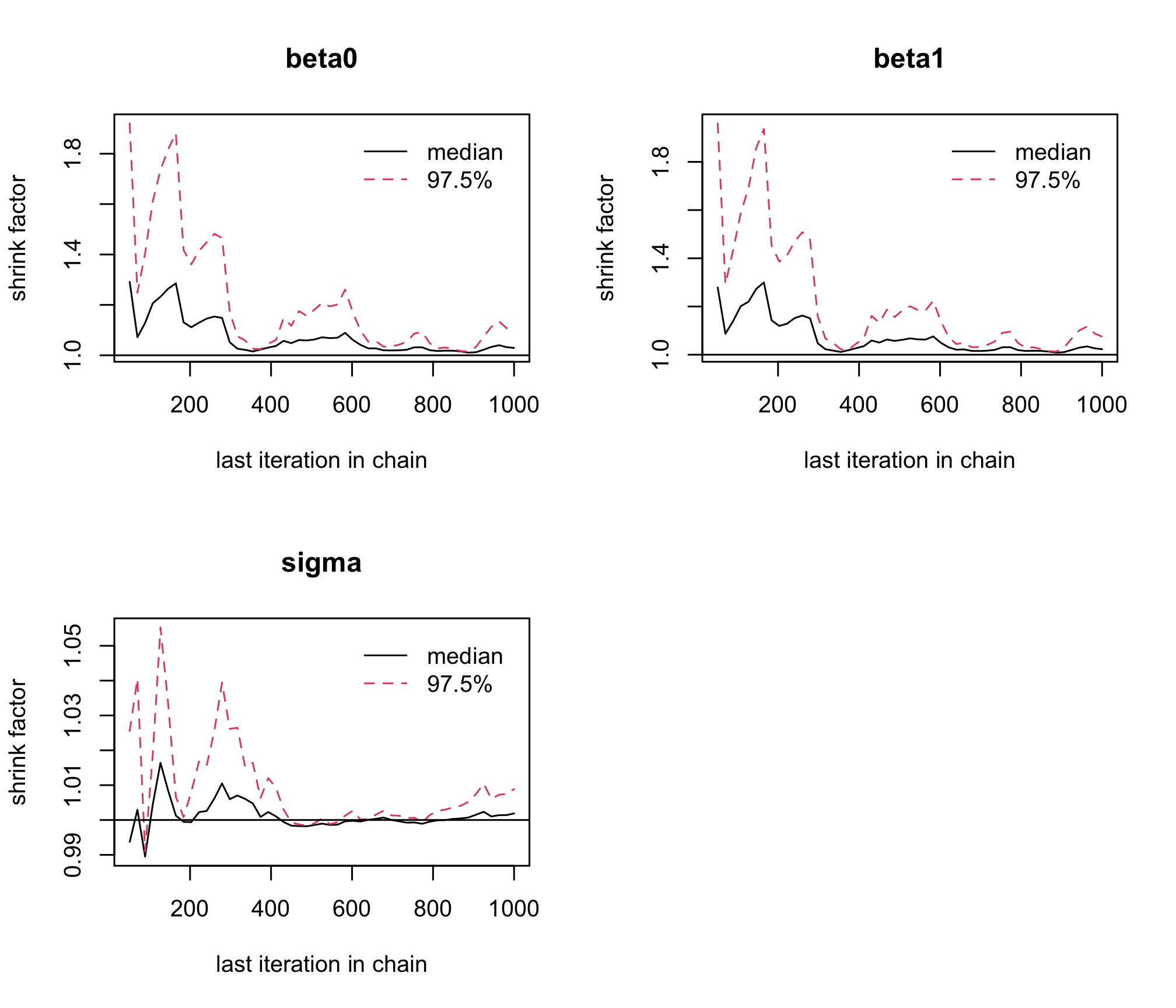
acfplot(res_albi_fixed)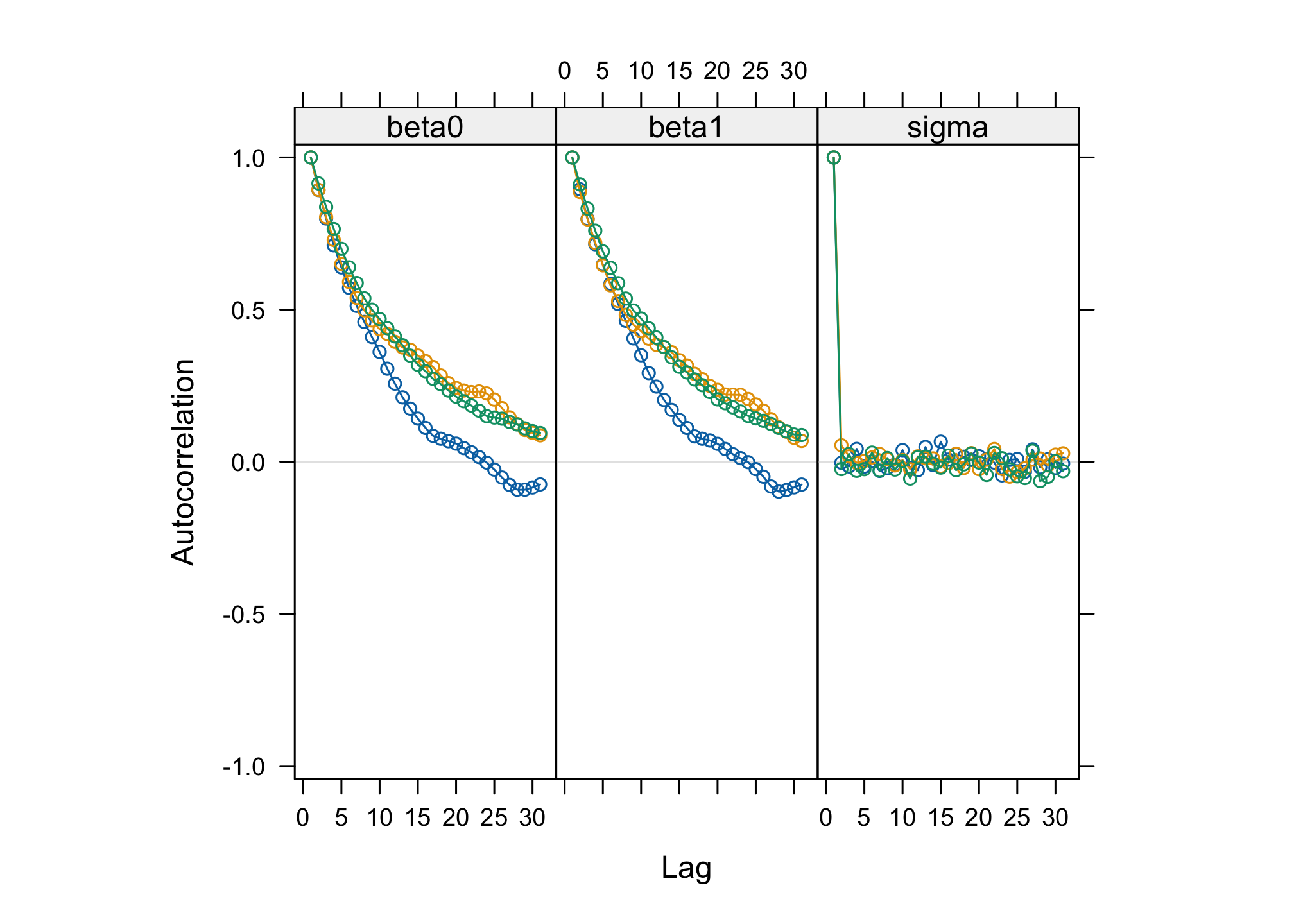
par(mfrow = c(3, 3)) cumuplot(res_albi_fixed, ask = FALSE, auto.layout = FALSE)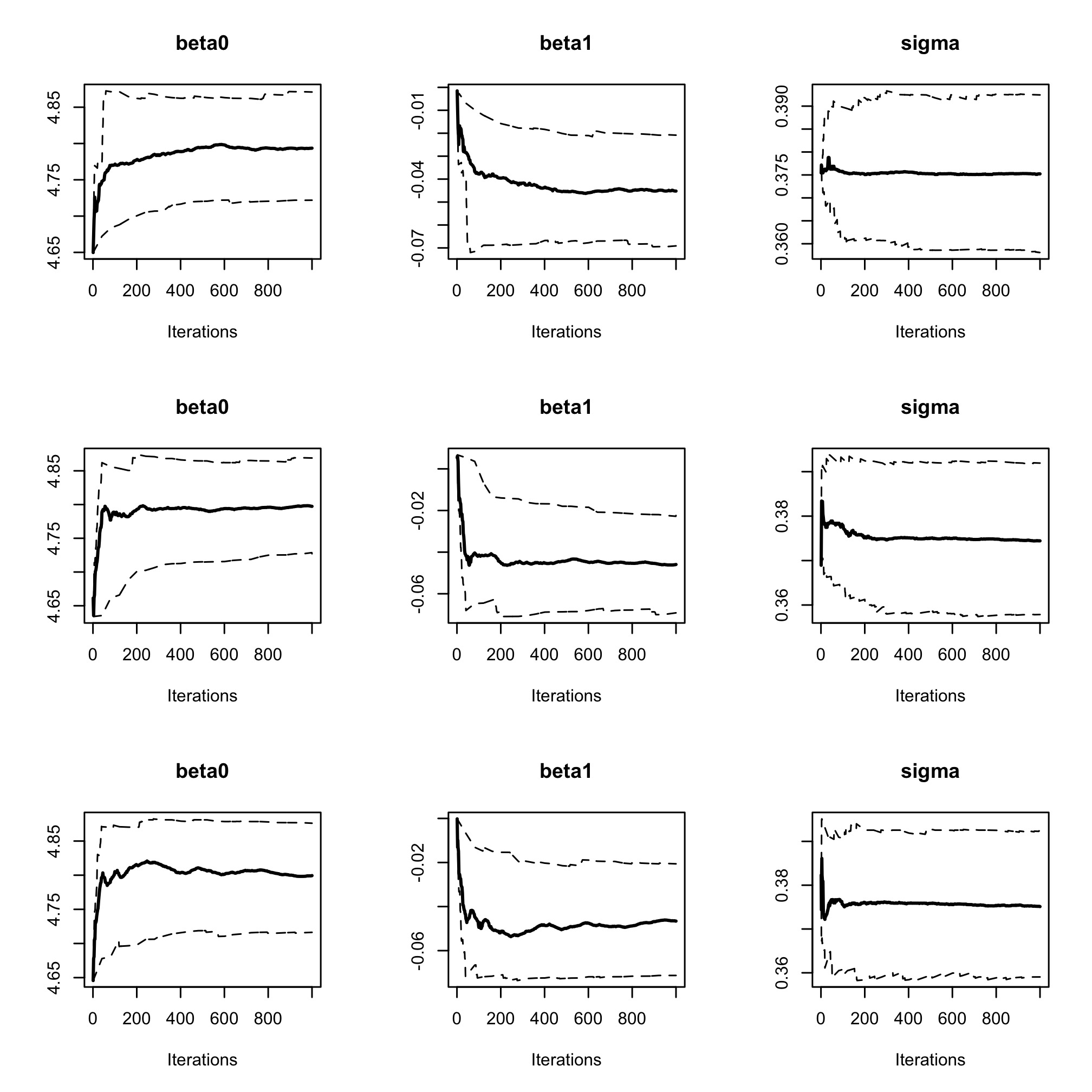
Using the
windowfunction, remove the 500 first iterations as burn-in to reach Markov Chain convergence to its stationary distribution (the posterior). Is it sufficient to solve convergence issues ?res_albi_fixed2 <- window(res_albi_fixed, start = 501) gelman.plot(res_albi_fixed2)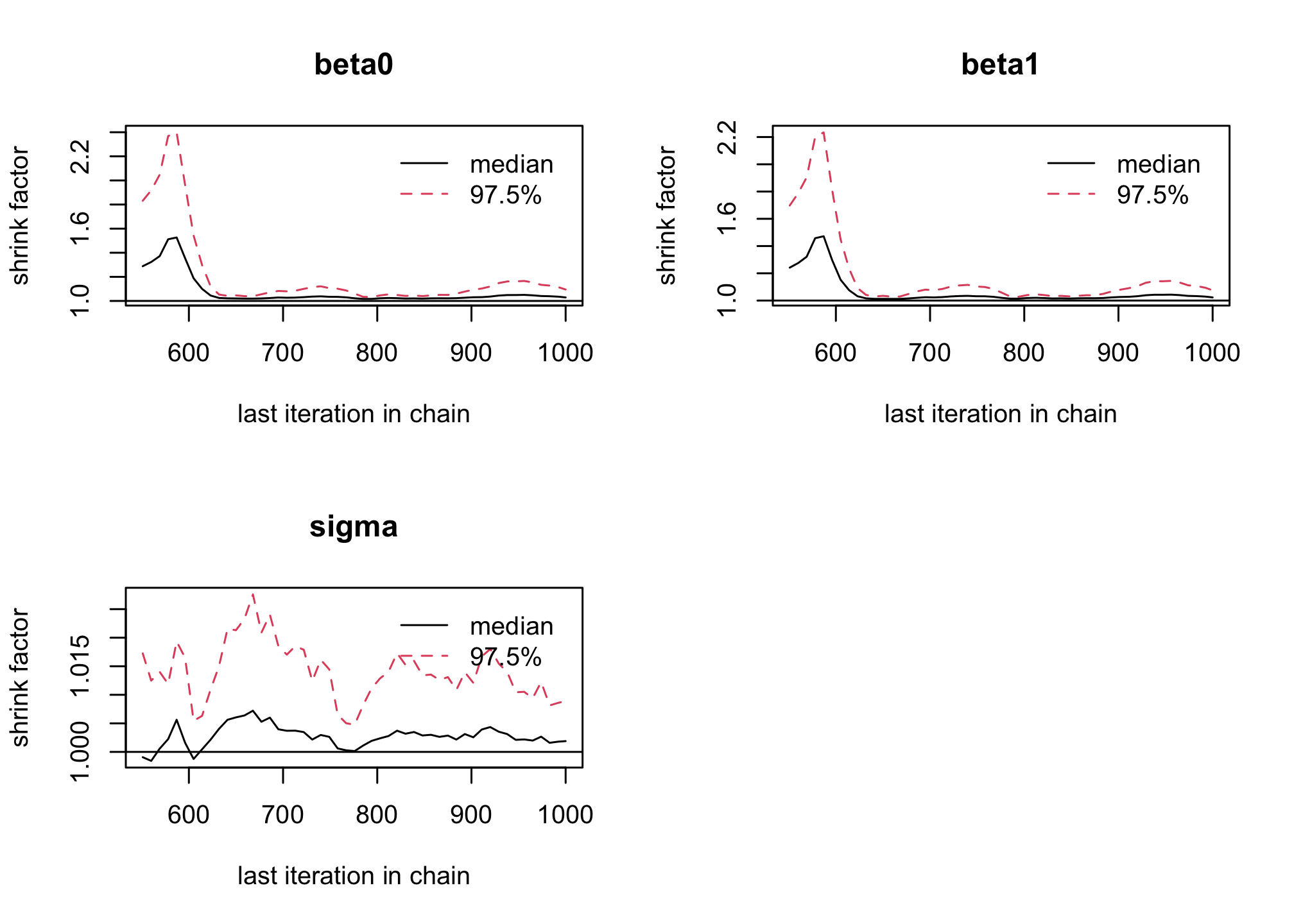
acfplot(res_albi_fixed2)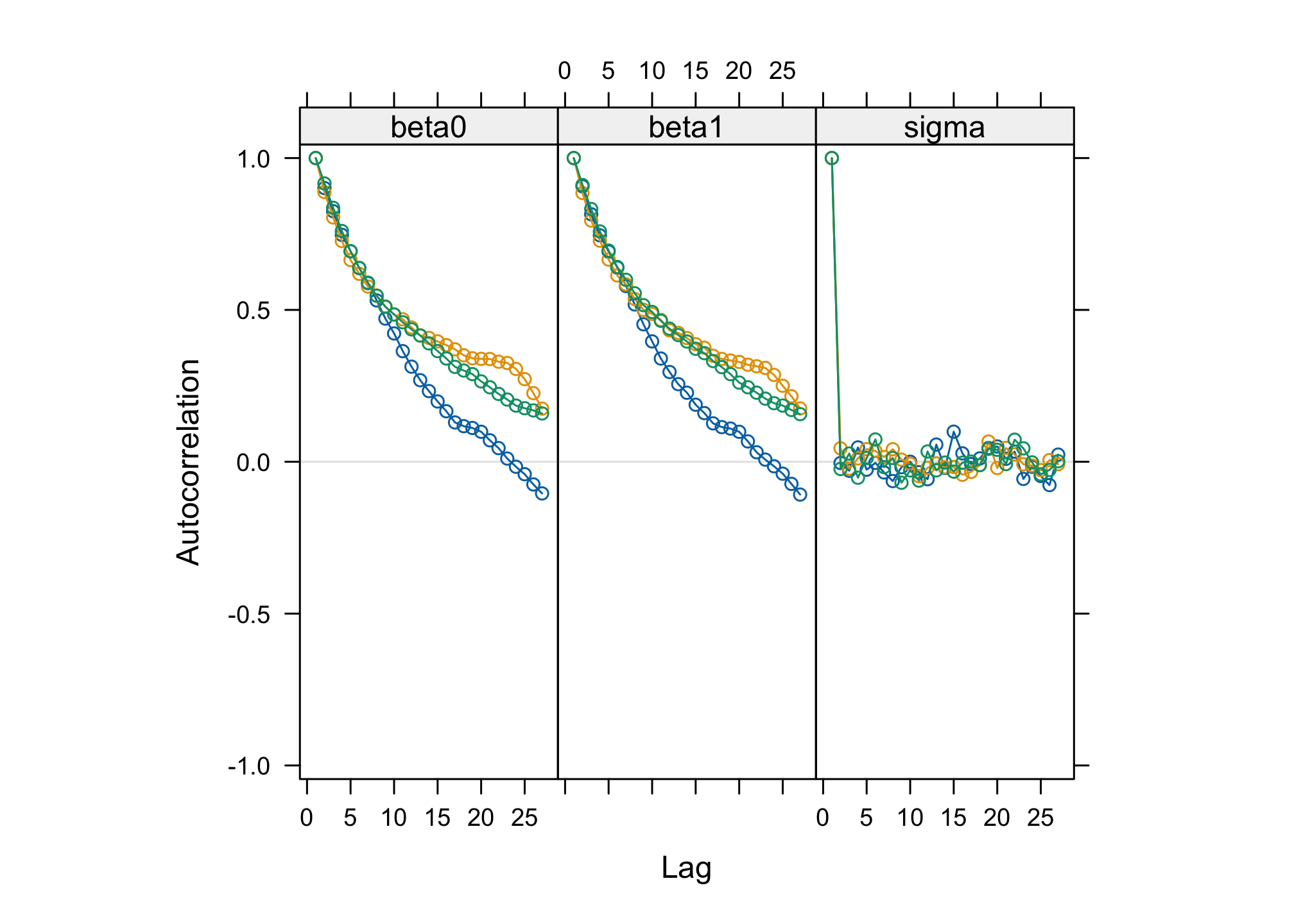
Check the effective sample size of the Monte Carlo sample with the
effectiveSize()function. Reduuce auto-corrlation by increasing thethinparameter to 10 incoda.samples. Check the impact on the effective sample`?`(effectiveSize) effectiveSize(res_albi_fixed2)## beta0 beta1 sigma ## 67.64399 78.03222 1500.00000albi_fixed_jags <- jags.model("albiBUGSmodel_fixed.txt", data = list(CD4t = albi$CD4t, N = nrow(albi), VL = albi$ViralLoad), n.chains = 3)## Compiling model graph ## Resolving undeclared variables ## Allocating nodes ## Graph information: ## Observed stochastic nodes: 950 ## Unobserved stochastic nodes: 3 ## Total graph size: 3195 ## ## Initializing modelres_albi_fixed_thin <- coda.samples(model = albi_fixed_jags, variable.names = c("beta0", "beta1", "sigma"), n.iter = 10000, thin = 10) res_albi_fixed_thin <- window(res_albi_fixed_thin, start = 5001) effectiveSize(res_albi_fixed_thin)## beta0 beta1 sigma ## 856.6286 901.3153 1763.5395plot(res_albi_fixed_thin)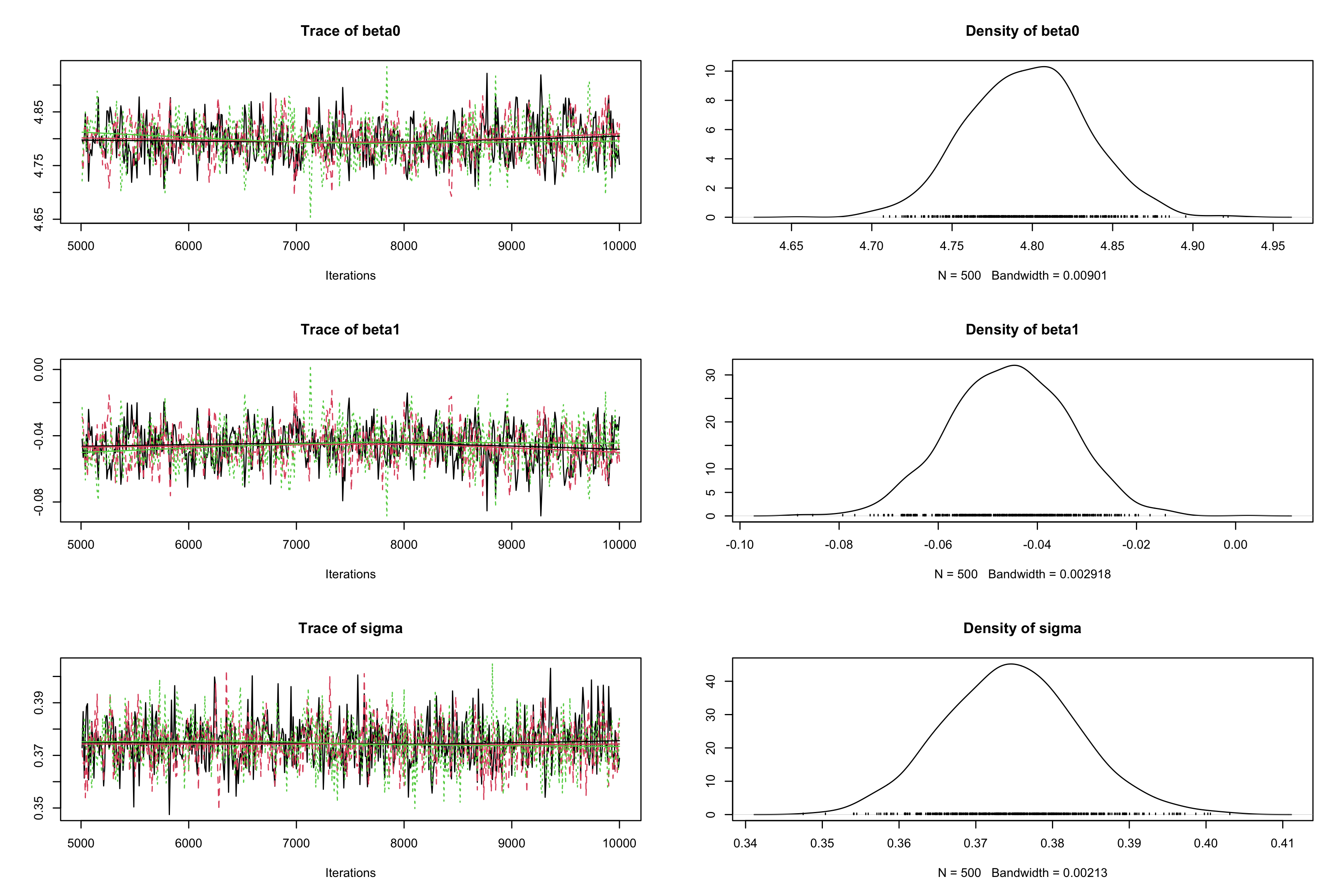
gelman.plot(res_albi_fixed_thin)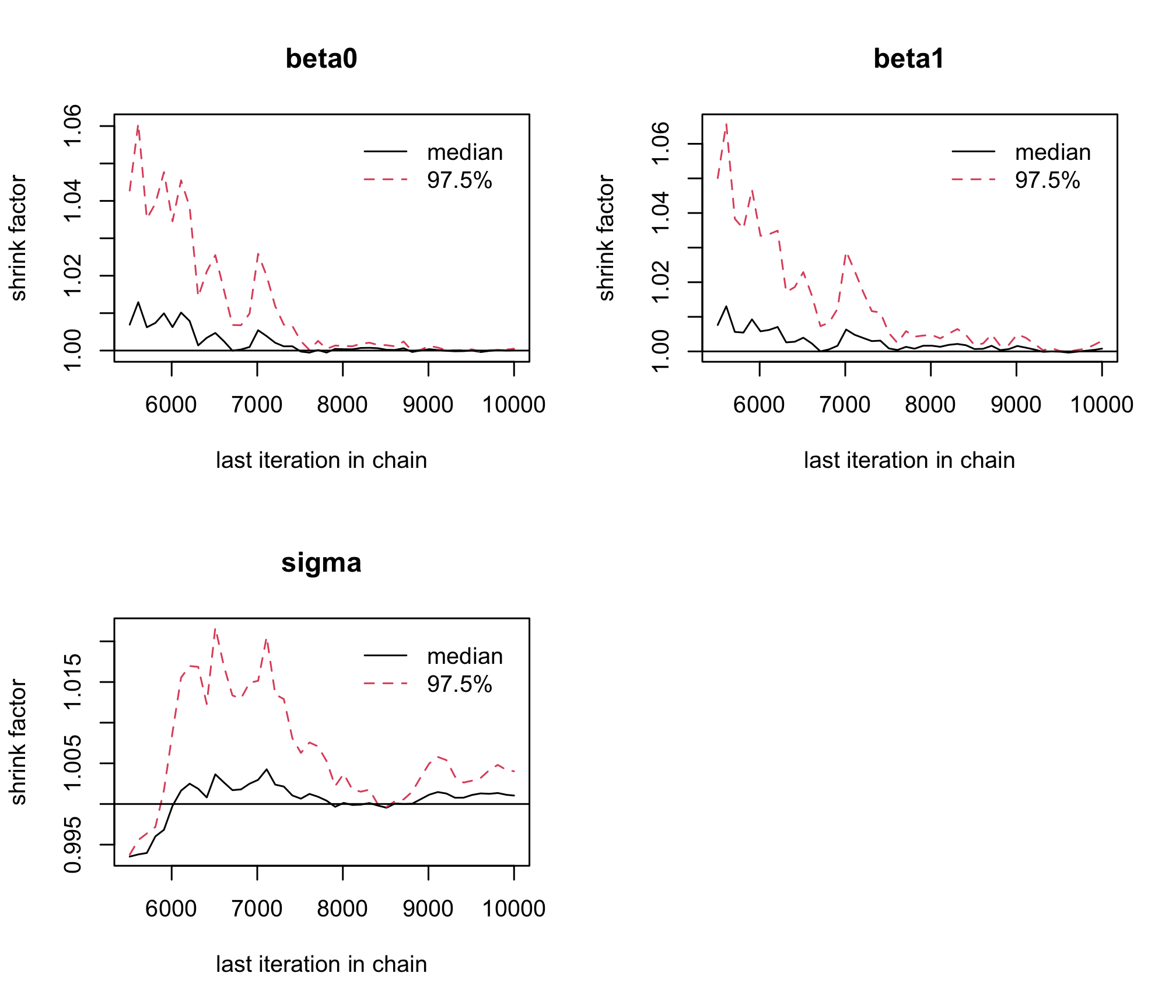
acfplot(res_albi_fixed_thin)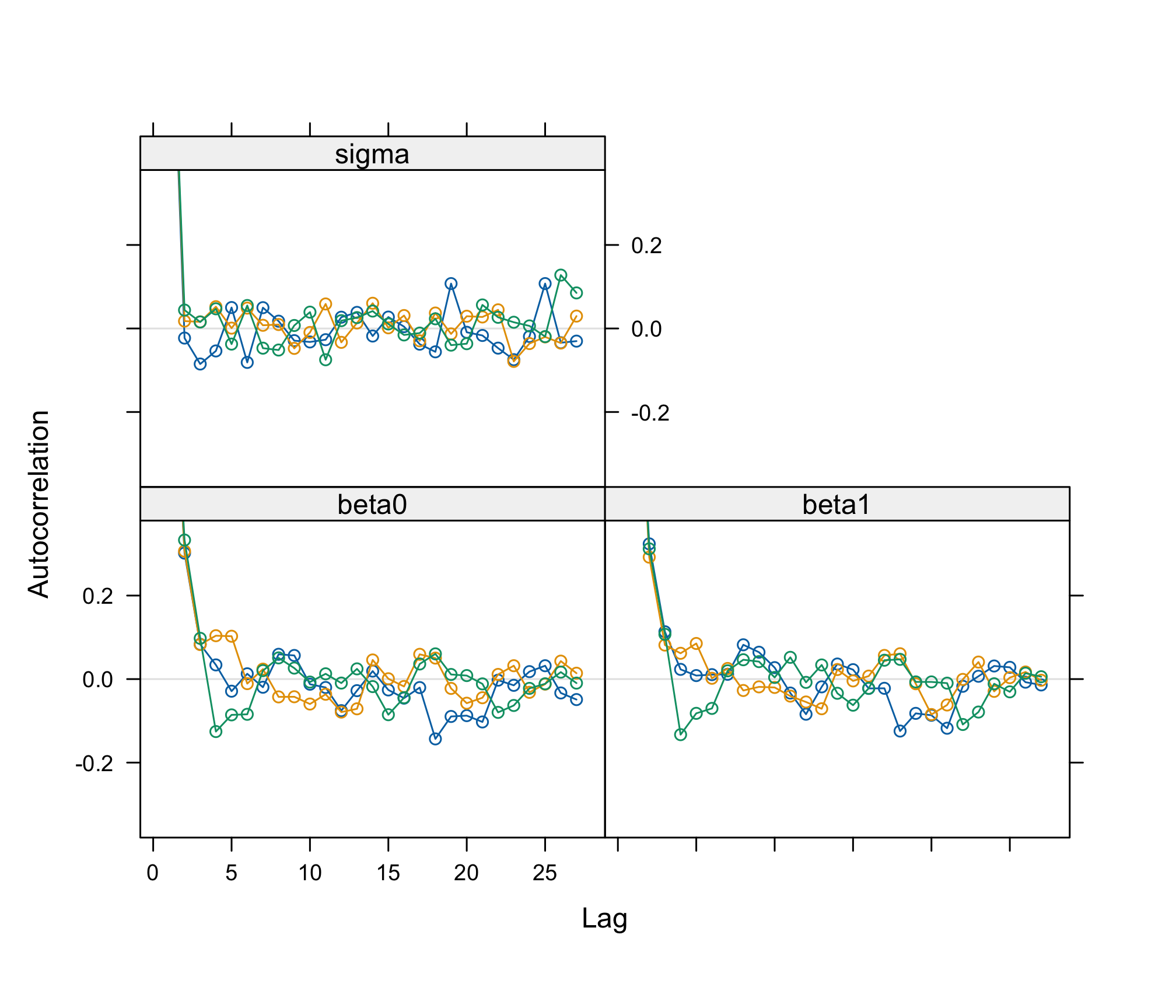
par(mfrow = c(3, 3)) cumuplot(res_albi_fixed_thin, ask = FALSE, auto.layout = FALSE)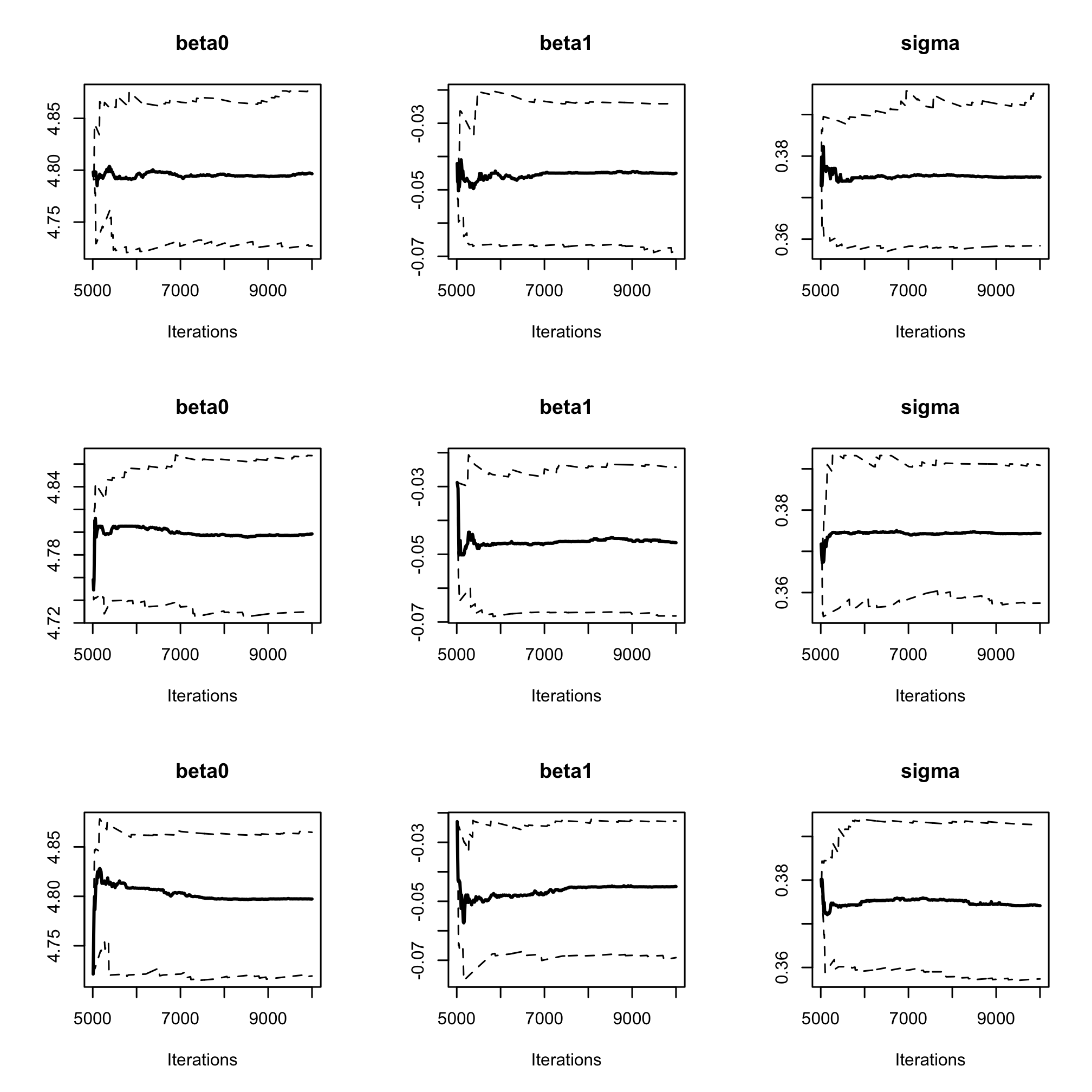
summary(res_albi_fixed_thin)## ## Iterations = 5010:10000 ## Thinning interval = 10 ## Number of chains = 3 ## Sample size per chain = 500 ## ## 1. Empirical mean and standard deviation for each variable, ## plus standard error of the mean: ## ## Mean SD Naive SE Time-series SE ## beta0 4.79694 0.036697 0.0009475 0.0012718 ## beta1 -0.04576 0.011885 0.0003069 0.0003997 ## sigma 0.37460 0.008777 0.0002266 0.0002131 ## ## 2. Quantiles for each variable: ## ## 2.5% 25% 50% 75% 97.5% ## beta0 4.72617 4.77143 4.79754 4.82145 4.86969 ## beta1 -0.06838 -0.05382 -0.04554 -0.03751 -0.02387 ## sigma 0.35761 0.36862 0.37443 0.38025 0.39236
Compare those results with a frequentist analysis.
summary(lm(CD4t ~ ViralLoad, data = albi))## ## Call: ## lm(formula = CD4t ~ ViralLoad, data = albi) ## ## Residuals: ## Min 1Q Median 3Q Max ## -1.0828 -0.2604 -0.0197 0.2449 1.3856 ## ## Coefficients: ## Estimate Std. Error t value Pr(>|t|) ## (Intercept) 4.79635 0.03696 129.8 < 2e-16 *** ## ViralLoad -0.04564 0.01201 -3.8 0.000154 *** ## --- ## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1 ## ## Residual standard error: 0.3742 on 948 degrees of freedom ## Multiple R-squared: 0.01501, Adjusted R-squared: 0.01397 ## F-statistic: 14.44 on 1 and 948 DF, p-value: 0.0001537Perform a Bayesian logistic regression to study the impact of the treatment on the binary outcome
CD4sup500adjusted on the viral load. Once you have a first estimation, try adding an interaction between the treatment and the viral load.# Fixed effect logistic regression Albi-ANRS model{ # likelihood for (i in 1:N){ CD4sup500[i] ~ dbern(proba[i]) logit(proba[i]) <- beta0 + beta1*VL[i] + beta2*Tt_switch[i] + beta3*d4t[i] + beta4*VL[i]*d4t[i] + beta5*VL[i]*Tt_switch[i] } #Priors beta0~dnorm(0,0.0001) beta1~dnorm(0,0.0001) beta2~dnorm(0,0.0001) beta3~dnorm(0,0.0001) beta4~dnorm(0,0.0001) beta5~dnorm(0,0.0001) #ORs OR_VL <- exp(beta1) OR_switch <- exp(beta2) OR_d4t <- exp(beta3) OR_d4tVL <- exp(beta4) OR_swVL <- exp(beta5) }library(rjags) albi_logis_jags <- jags.model("albiBUGSmodel_logistic_fixed.txt", data = list(CD4sup500 = 1 * (albi$CD4sup500 == "Yes"), N = nrow(albi), VL = albi$ViralLoad, d4t = 1 * (albi$Treatment == "d4t+ddI"), Tt_switch = 1 * (albi$Treatment == "switch")), n.chains = 3)## Compiling model graph ## Resolving undeclared variables ## Allocating nodes ## Graph information: ## Observed stochastic nodes: 950 ## Unobserved stochastic nodes: 6 ## Total graph size: 7299 ## ## Initializing modelres_albi_logis <- coda.samples(model = albi_logis_jags, variable.names = c("OR_VL", "OR_switch", "OR_d4t", "OR_d4tVL", "OR_swVL"), n.iter = 10000) res_albi_logis_burnt <- window(res_albi_logis, start = 5001) plot(res_albi_logis_burnt)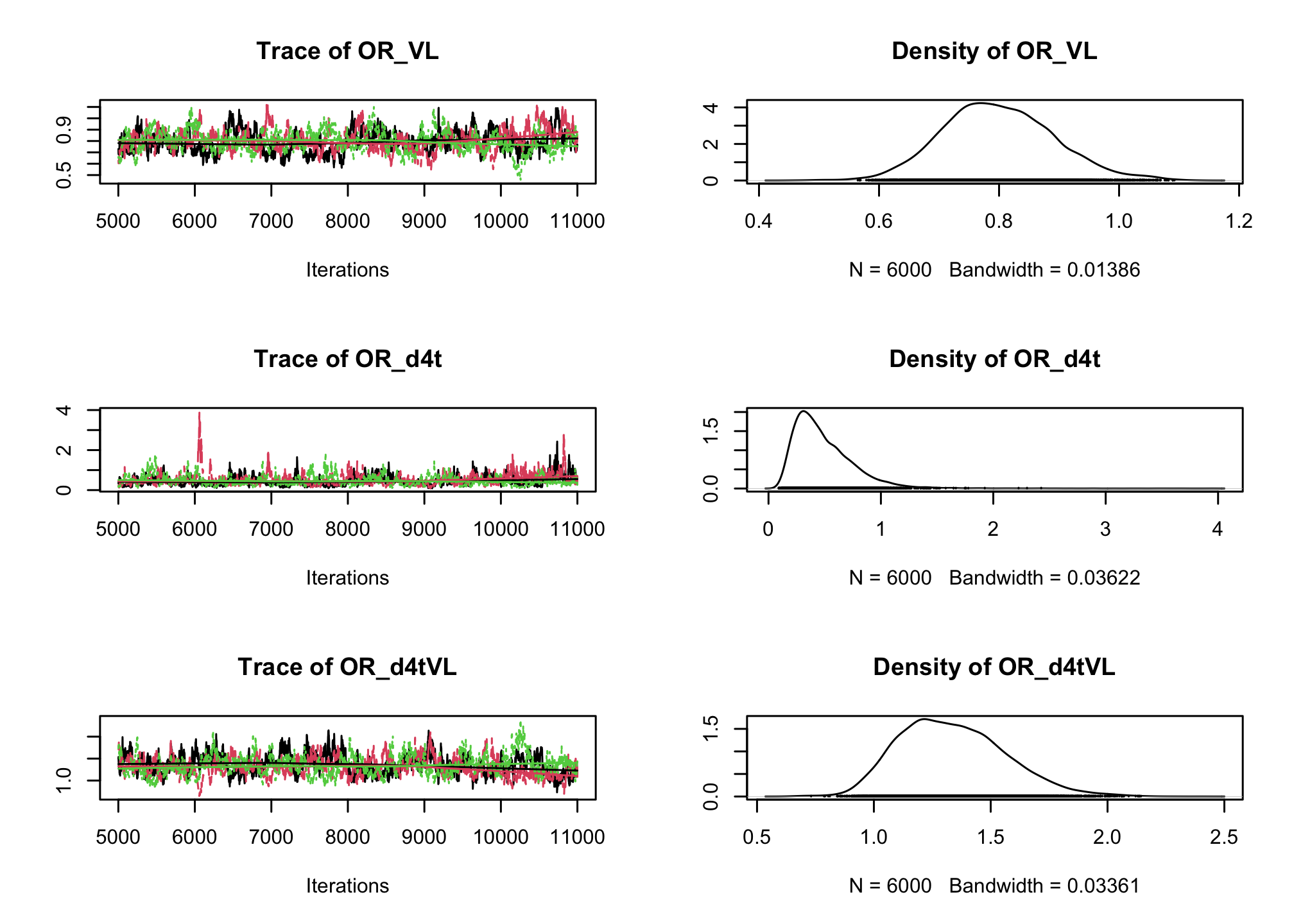
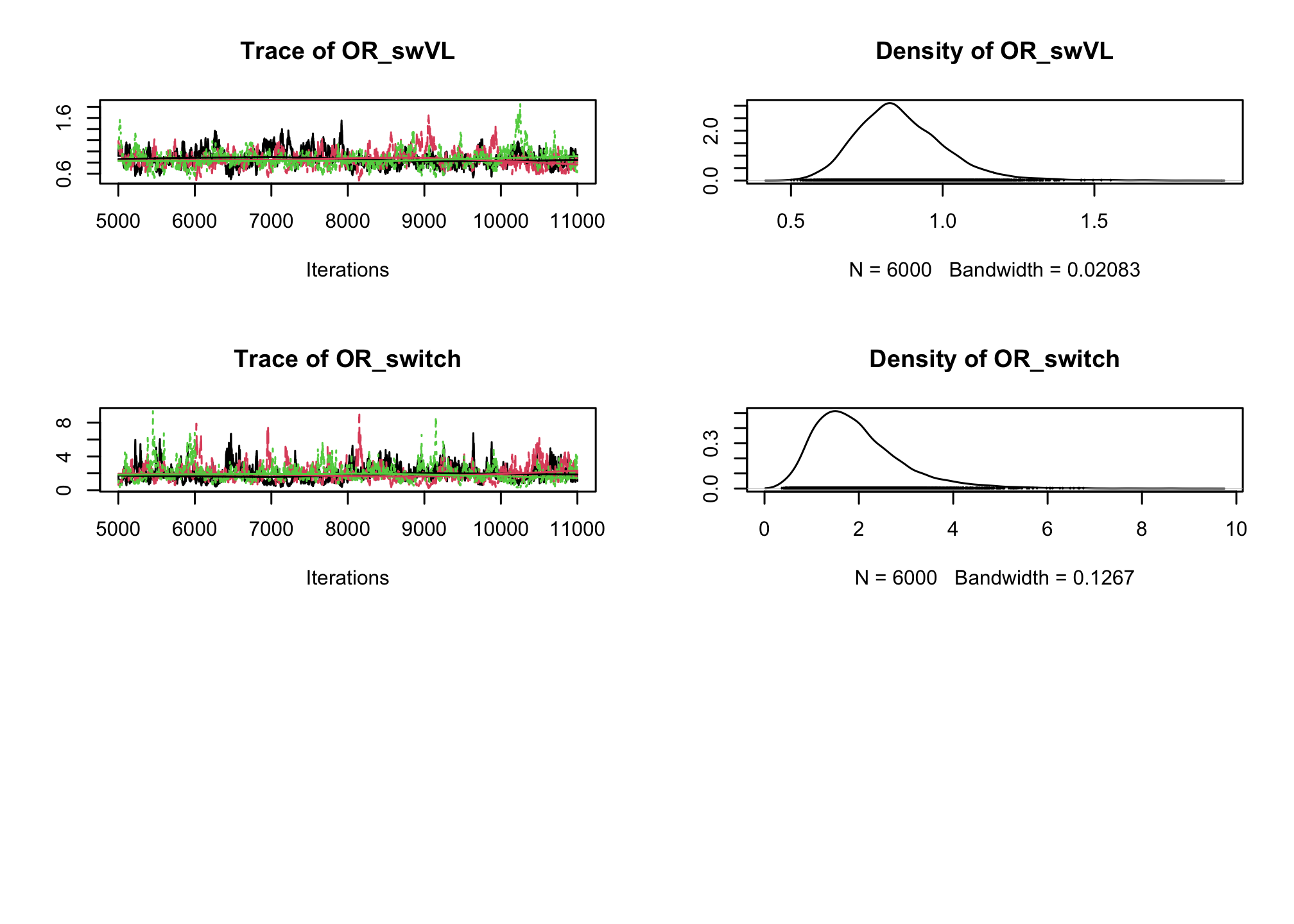
summary(res_albi_logis_burnt)## ## Iterations = 5001:11000 ## Thinning interval = 1 ## Number of chains = 3 ## Sample size per chain = 6000 ## ## 1. Empirical mean and standard deviation for each variable, ## plus standard error of the mean: ## ## Mean SD Naive SE Time-series SE ## OR_VL 0.7991 0.09281 0.0006917 0.006895 ## OR_d4t 0.4911 0.27675 0.0020628 0.017052 ## OR_d4tVL 1.3364 0.22505 0.0016774 0.016196 ## OR_swVL 0.8644 0.15089 0.0011247 0.009802 ## OR_switch 1.9715 0.96411 0.0071861 0.053425 ## ## 2. Quantiles for each variable: ## ## 2.5% 25% 50% 75% 97.5% ## OR_VL 0.6285 0.7350 0.7947 0.8596 0.9947 ## OR_d4t 0.1582 0.2970 0.4281 0.6219 1.1295 ## OR_d4tVL 0.9597 1.1715 1.3177 1.4825 1.8159 ## OR_swVL 0.6192 0.7618 0.8455 0.9487 1.2081 ## OR_switch 0.6461 1.2996 1.7906 2.4361 4.3859summary(glm(CD4sup500 == "Yes" ~ ViralLoad * Treatment, family = "binomial", data = albi))## ## Call: ## glm(formula = CD4sup500 == "Yes" ~ ViralLoad * Treatment, family = "binomial", ## data = albi) ## ## Coefficients: ## Estimate Std. Error z value Pr(>|z|) ## (Intercept) 0.3279 0.3241 1.012 0.3117 ## ViralLoad -0.2556 0.1197 -2.135 0.0328 * ## Treatmentd4t+ddI -0.9409 0.5338 -1.763 0.0779 . ## Treatmentswitch 0.4836 0.4807 1.006 0.3143 ## ViralLoad:Treatmentd4t+ddI 0.3084 0.1737 1.776 0.0757 . ## ViralLoad:Treatmentswitch -0.1303 0.1690 -0.771 0.4409 ## --- ## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1 ## ## (Dispersion parameter for binomial family taken to be 1) ## ## Null deviance: 1287.8 on 949 degrees of freedom ## Residual deviance: 1270.9 on 944 degrees of freedom ## AIC: 1282.9 ## ## Number of Fisher Scoring iterations: 4So far we have ignored the longitudinal nature of our measurements. Now, lets add a random intercept in our logistic regression to account for the intra-patient correlation across visits.
ggplot(albi, aes(y = CD4, x = Day, color = Treatment)) + geom_hline(aes(yintercept = 500), color = "grey35", linetype = 2) + geom_line(aes(group = PatientID), alpha = 0.3) + MetBrewer::scale_color_met_d("Java") + theme_bw()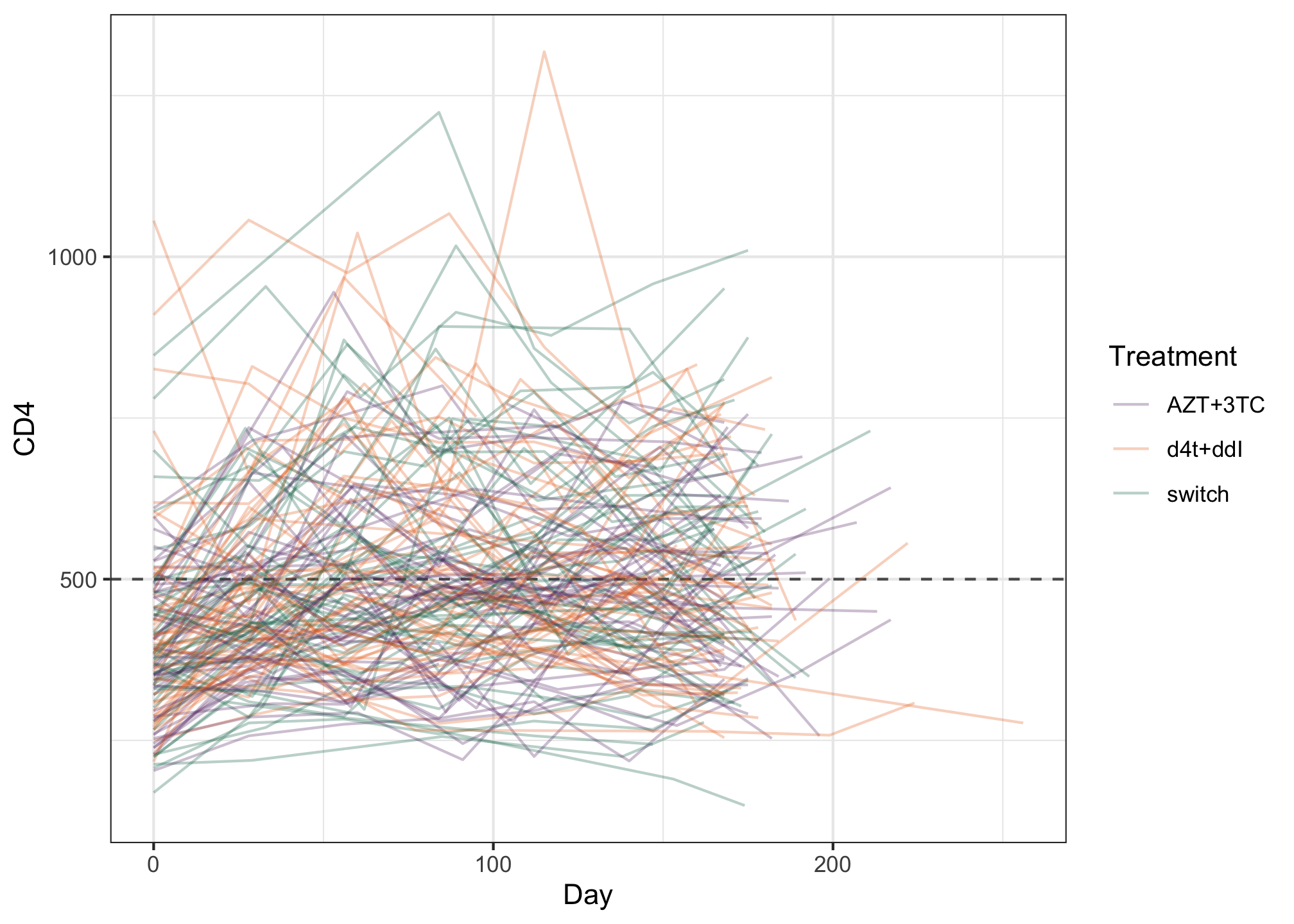
# Mixed effect logistic regression Albi-ANRS model{ # likelihood for (i in 1:N){ CD4sup500[i] ~ dbern(proba[i]) logit(proba[i]) <- beta0 + b0[PAT_ID[i]] + beta1*VL[i] + beta2*Tt_switch[i] + beta3*d4t[i] + beta4*VL[i]*d4t[i] + beta5*VL[i]*Tt_switch[i] } for (j in 1:Npat){ b0[j]~dnorm(0, tau_b0) } #Priors beta0~dnorm(0,0.0001) beta1~dnorm(0,0.0001) beta2~dnorm(0,0.0001) beta3~dnorm(0,0.0001) beta4~dnorm(0,0.0001) beta5~dnorm(0,0.0001) tau_b0~dgamma(0.001,0.001) #ORs OR_VL <- exp(beta1) OR_switch <- exp(beta2) OR_d4t <- exp(beta3) OR_d4tVL <- exp(beta4) OR_swVL <- exp(beta5) sigma_RI <- pow(tau_b0, -0.5) }library(rjags) albi_logis_RI_jags <- jags.model("albiBUGSmodel_logistic_RandomInt.txt", data = list(CD4sup500 = 1 * (albi$CD4sup500 == "Yes"), N = nrow(albi), VL = albi$ViralLoad, d4t = 1 * (albi$Treatment == "d4t+ddI"), Tt_switch = 1 * (albi$Treatment == "switch"), Npat = length(levels(albi$PatientID)), PAT_ID = as.numeric(albi$PatientID)), n.chains = 3)## Compiling model graph ## Resolving undeclared variables ## Allocating nodes ## Graph information: ## Observed stochastic nodes: 950 ## Unobserved stochastic nodes: 156 ## Total graph size: 8670 ## ## Initializing modelres_albi_logis_RI <- coda.samples(model = albi_logis_RI_jags, variable.names = c("OR_VL", "OR_switch", "OR_d4t", "OR_d4tVL", "OR_swVL", "sigma_RI"), n.iter = 10000) res_albi_logis_RI_burnt <- window(res_albi_logis_RI, start = 1001) plot(res_albi_logis_RI_burnt)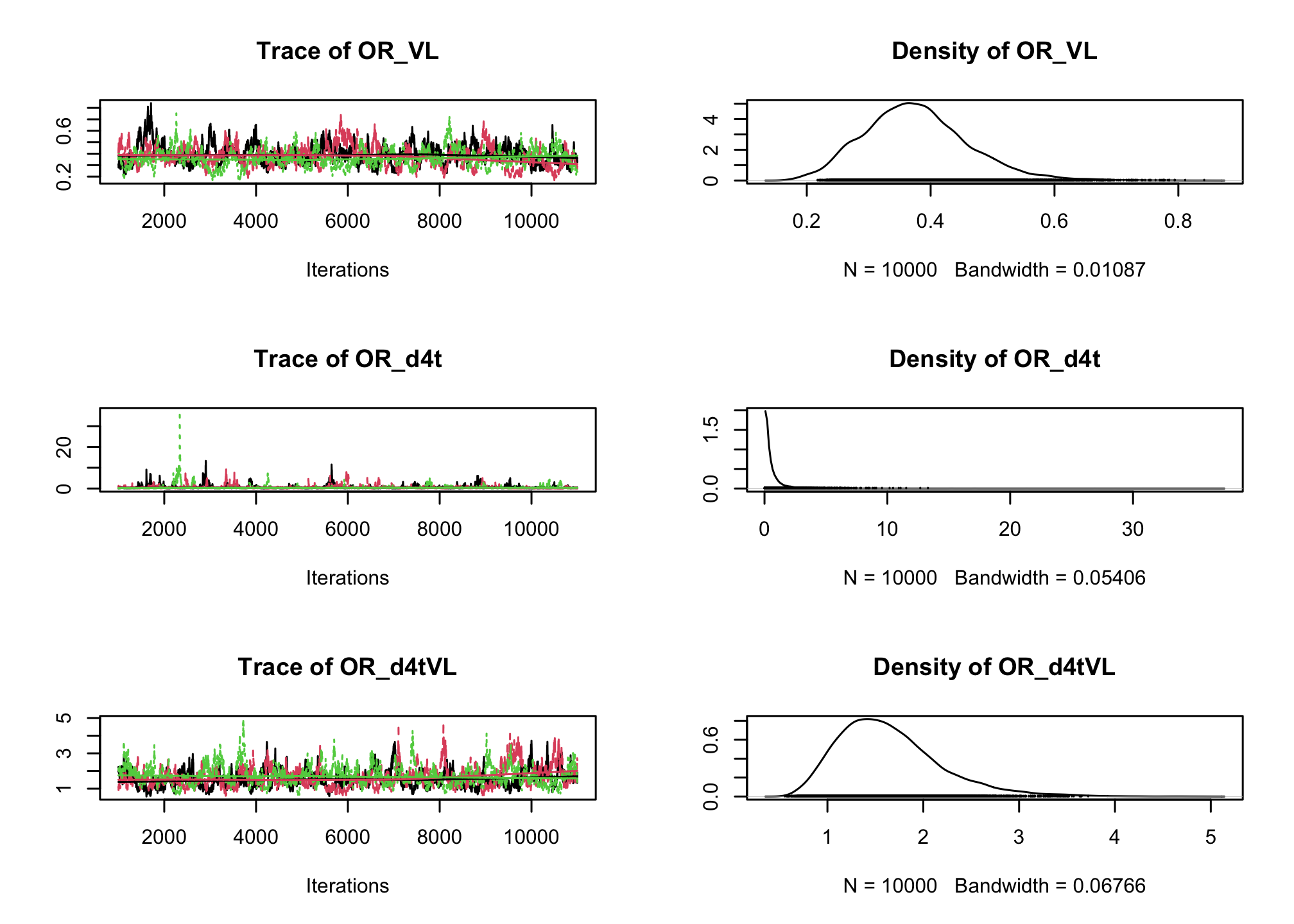
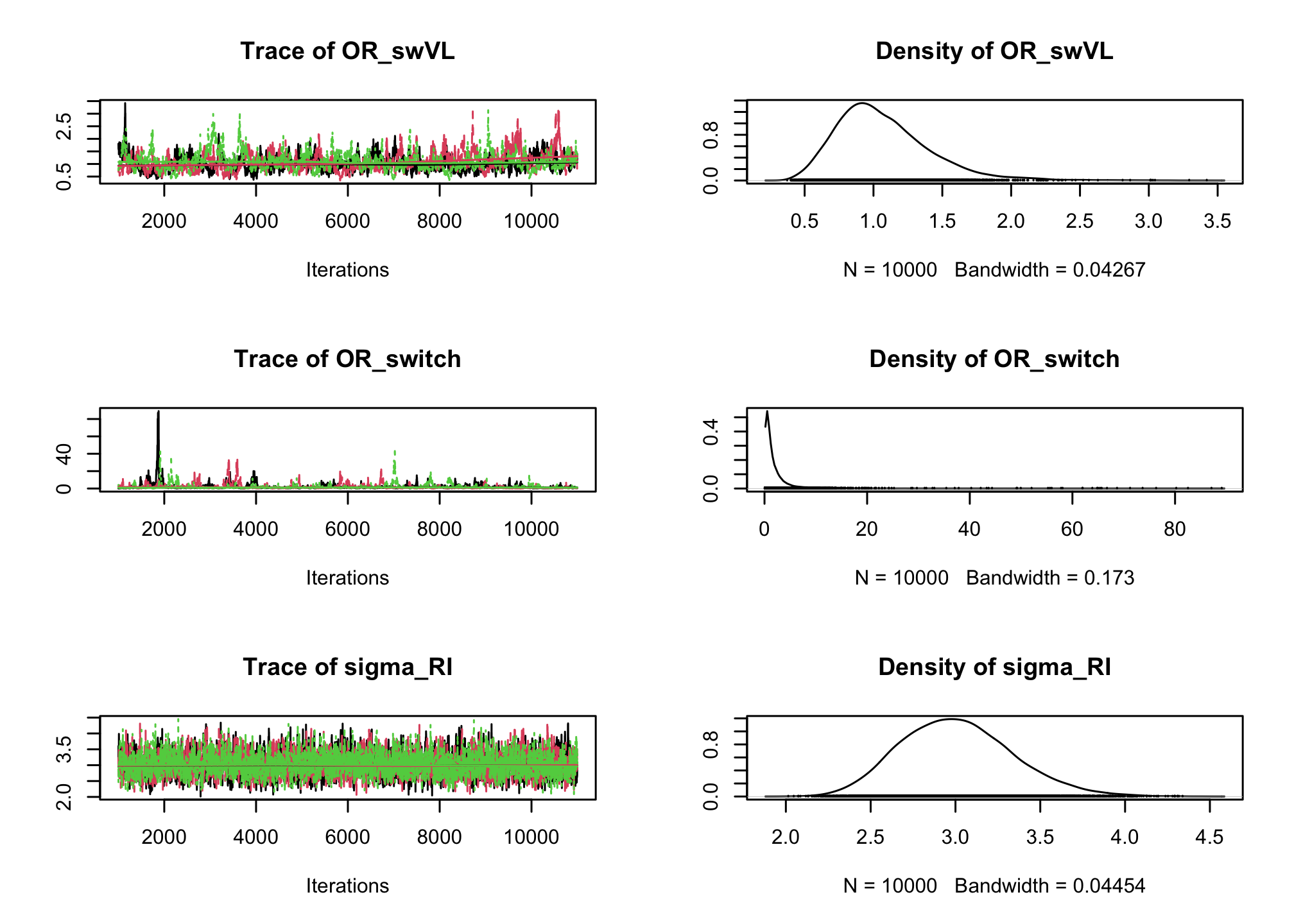
summary(res_albi_logis_RI_burnt)## ## Iterations = 1001:11000 ## Thinning interval = 1 ## Number of chains = 3 ## Sample size per chain = 10000 ## ## 1. Empirical mean and standard deviation for each variable, ## plus standard error of the mean: ## ## Mean SD Naive SE Time-series SE ## OR_VL 0.3768 0.0844 0.0004873 0.005884 ## OR_d4t 0.6035 1.0108 0.0058357 0.056536 ## OR_d4tVL 1.6451 0.5374 0.0031024 0.033932 ## OR_swVL 1.0694 0.3438 0.0019849 0.020211 ## OR_switch 2.0137 3.4572 0.0199603 0.181254 ## sigma_RI 3.0094 0.3303 0.0019069 0.006740 ## ## 2. Quantiles for each variable: ## ## 2.5% 25% 50% 75% 97.5% ## OR_VL 0.23183 0.3190 0.3710 0.4269 0.5632 ## OR_d4t 0.03263 0.1413 0.3105 0.6785 3.0004 ## OR_d4tVL 0.84315 1.2601 1.5666 1.9324 2.9406 ## OR_swVL 0.55391 0.8284 1.0156 1.2524 1.8851 ## OR_switch 0.12952 0.5262 1.0706 2.2452 9.5853 ## sigma_RI 2.42311 2.7730 2.9922 3.2210 3.7080library(lme4) summary(lme4::glmer(CD4sup500 == "Yes" ~ (1 | PatientID) + ViralLoad * Treatment, family = "binomial", data = albi))## Generalized linear mixed model fit by maximum likelihood (Laplace ## Approximation) [glmerMod] ## Family: binomial ( logit ) ## Formula: CD4sup500 == "Yes" ~ (1 | PatientID) + ViralLoad * Treatment ## Data: albi ## ## AIC BIC logLik deviance df.resid ## 971.8 1005.8 -478.9 957.8 943 ## ## Scaled residuals: ## Min 1Q Median 3Q Max ## -2.9273 -0.4021 -0.1947 0.4119 3.9688 ## ## Random effects: ## Groups Name Variance Std.Dev. ## PatientID (Intercept) 7.317 2.705 ## Number of obs: 950, groups: PatientID, 149 ## ## Fixed effects: ## Estimate Std. Error z value Pr(>|z|) ## (Intercept) 1.901075 0.689951 2.755 0.00586 ** ## ViralLoad -0.955590 0.212725 -4.492 7.05e-06 *** ## Treatmentd4t+ddI -1.177377 1.103321 -1.067 0.28592 ## Treatmentswitch 0.101717 1.000221 0.102 0.91900 ## ViralLoad:Treatmentd4t+ddI 0.439794 0.308828 1.424 0.15443 ## ViralLoad:Treatmentswitch 0.008679 0.291170 0.030 0.97622 ## --- ## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1 ## ## Correlation of Fixed Effects: ## (Intr) VirlLd Trt4+I Trtmnt VL:T4+ ## ViralLoad -0.780 ## Trtmntd4t+I -0.616 0.473 ## Trtmntswtch -0.679 0.521 0.423 ## VrlLd:Tr4+I 0.518 -0.657 -0.821 -0.355 ## VrlLd:Trtmn 0.549 -0.695 -0.341 -0.786 0.475
Jean-Michel Molina et al. “The ALBI Trial: A Randomized Controlled Trial Comparing Stavudine Plus Didanosine with Zidovudine Plus Lamivudine and a Regimen Alternating Both Combinations in Previously Untreated Patients Infected with Human Immunodeficiency Virus,” The Journal of Infectious Diseases 180, no. 2 (1999): 351–358, doi:10.1086/314891.↩︎